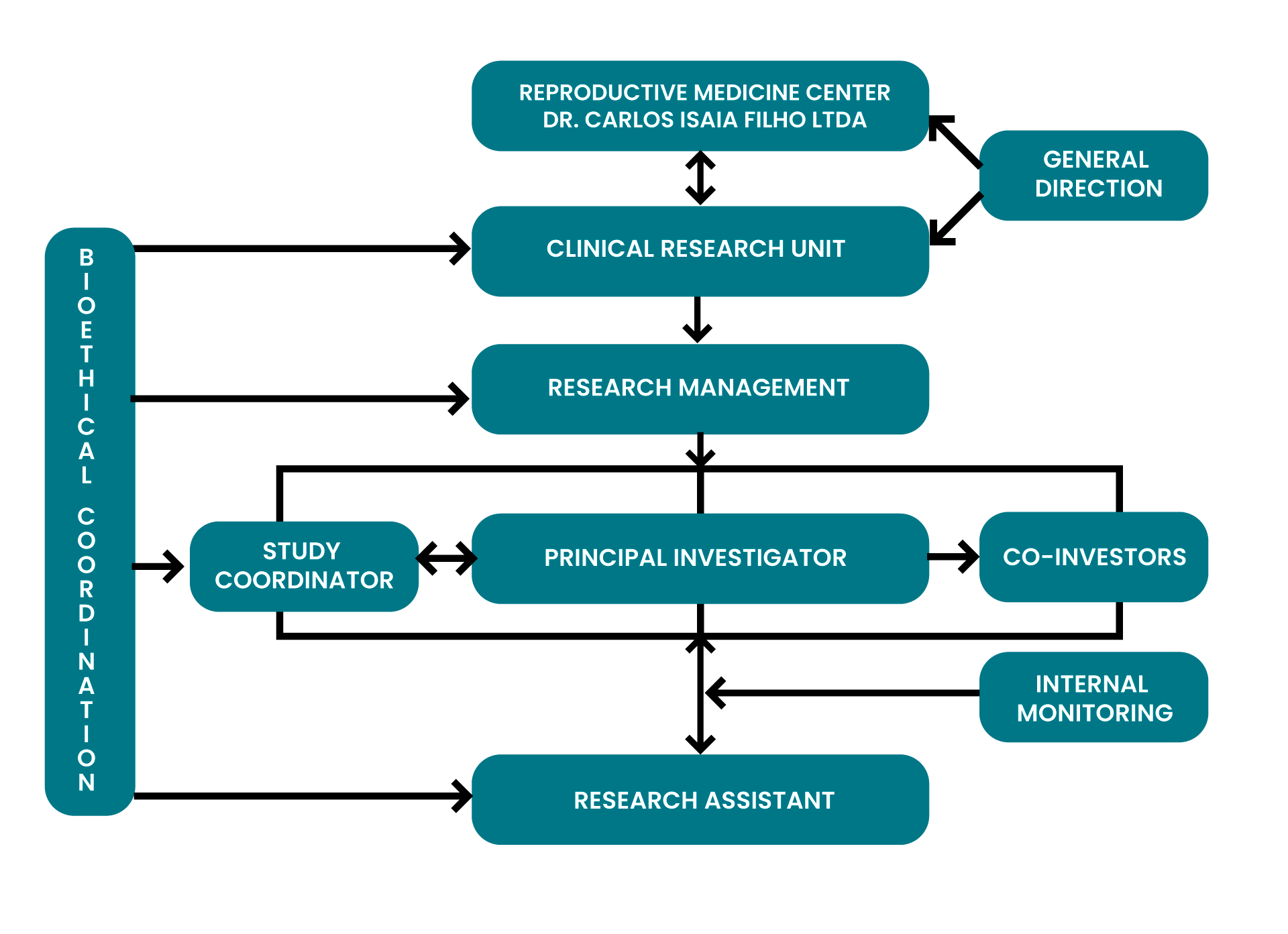

The Clinical Research Unit of the Isaia Institute is a private clinical research located in Porto Alegre, Brazil.
With over 40 years of experience, we offer infrastructure, advanced technology, logistics and a highly specialized team to support that allow us to conduct all stages of clinical trials under ideal conditions, strictly following the Good Clinical Practice (GCP) Manual, prepared by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), in addition to national regulations regulated by the National Health Council, the National Research Ethics Commission and the National Health Surveillance Agency/Ministry of Health.
Furthermore, due to the accumulated experience, since 2006 we have been acting as a Contract Research Organization (CRO), responding to requests from clinical trial sponsors.

In August 2004, we were audited by the US Food & Drug Administration (FDA) for conducting a multi-site trial, in which we were one of 54 centers involved, distributed across 12 countries. We have the highest inclusion rate and the lowest discontinuation rate of research participants. The audit confirmed full compliance with the agency’s requirements, reaffirming the quality of our work.
We have a multidisciplinary clinical research team, a group of associated clinical researchers from different specialties and services working in regulated technical partnership in the clinical, radiological, hospital and emergency areas.
 Dr. Carlos Isaia Filho
Physician
Dr. Carlos Isaia Filho
PhysicianPhysician, with a Bachelor’s Degree from the Federal University of Santa Maria in 1976, specializing in Gynecology and Obstetrics. He has extensive international experience, having been a Research Fellow of the World Health Organization at Withington Hospital, in Manchester (England), between 1978 and 1980, and a Research Fellow at Johns Hopkins University, in Baltimore (USA), in 1983. He is a specialist in Human Reproduction from UFRS(1990).
Throughout his career, he has played leadership roles in public health and invarious institutions. He was head of the Women’s Health Division at the Department of Health and Environment of the State of Rio Grande do Sul (1991-1998) and coordinator of the Maternal and Child unit at Hospital Mãe de Deus (1989-1993). He also served as a delegate for the Brazilian Society of Human Reproduction for Rio Grande do Sul (1984-1988) and held several prominent positions in the Brazilian Society of Laparoscopic Surgery and the Society of Gynecological Endoscopy of Rio Grande do Sul.
In addition, he was a health commentator on Jornal do Almoço on RBS-TV (1995-1998) and a member of the National Commission on Maternal Mortality of the Ministry of Health (1996-1998). During his career at the Regional Council of Medicine of Rio Grande do Sul (CREMERS), he served as a counselor and ombudsman from 2018 to 2023, and was chairman of the institution from 2020 to 2022.
 Anamaria Feijó
PhD in Bioethics
Anamaria Feijó
PhD in BioethicsShe holds a degree in Science, with a specialization in Biology, from the Pontifical Catholic University of Rio Grande do Sul (PUCRS) (1982). She completed her Master’s Degree in Education from the same institution (1995) and obtained her PhD in Philosophy, with an emphasis on Bioethics and Ethics Applied to Animals, from Universidad de Buenos Aires (2003). She was an assistant professor at the School of Biosciences at PUCRS from 1986 to 2015. Between 2007 and 2013, she served as Coordinator of the Ethics Committee for the Use of Animals (CEUA/PUCRS) and, from 2006 to 2015, she set up and coordinated the Laboratory of Bioethics and Ethics Applied to Animals at the Institute of Bioethics at PUCRS. She was also an advisor to the National Commission for the Control of Animal Experimentation (CONCEA), linked to the MCTI, from 2012 to 2015. She currently holds the position of Bioethics Managerand Research Assistant at the Dr. Carlos Isaia Filho Clinical Research Unit.
 Andréa Moura G. Locatelli
Physician
Andréa Moura G. Locatelli
Physician Bachelor’s Degree in Medicine from the Federal University of Rio Grande (1994), she completed her Medical Residency at the Dr. Miguel Riet Corrêa Jr. University Hospital (1996). She is a gynecologist and obstetrician.
 Eliane A. H. do Amaral
Physician
Eliane A. H. do Amaral
Physician 
Bachelor’s degree in Medicine from the Federal University of Santa Maria, RS (1983). She completed her Medical Residency in Gynecology and Obstetrics at the University of Passo Fundo, at the São Vicente de Paulo Hospital, Passo Fundo, RS, from 1984 to 1986. She has a specialization in Clinical Sexology from FUMEC University, in Belo Horizonte, MG, from 2003 to 2004. Additionally, she is a member of the International Society for Sexual Medicine.
 Bárbara Feijó Wünsch
Biologist
Bárbara Feijó Wünsch
Biologist
Graduated in Biological Sciences, with a Bachelor’s Degree from the Pontifical Catholic University of Rio Grande do Sul. She has experience as a Laboratory Technician with Mesenchymal Stem Cells at CellMed Regenerative Veterinary Medicine and Scientific Consulting, from October 2015 to September 2017. She holds a Master’s degree from the Postgraduate Program in Pathology at UFCSPA.
 Iandara Silva Neto
Biomedical
Iandara Silva Neto
BiomedicalGraduated in Biomedicine from the Federal University of Health Sciences of Porto Alegre (UFCSPA), with experience in clinical analysis and pharmacology. Currently works as a clinical study coordinator and study monitor at the Clinical Research Unit of the Isaia Institute since September 2025.
Carlos Roberto Maia (Gynecologist and Obstetrician/Member of the Brazilian College of Radiology and Diagnostic Imaging);
Ignácio Osorio Mallmann (Coloproctologist);
Jorge Ilha Guimarães (Cardiologist);
José Henrique Muller (Otorhinolaryngologist).
HOSPITALS
Hospital São Lucas da PUCRS - Emergency Service*.
* for emergency care of research participants
LABORATORIES
Mont’serrat Laboratory – Clinical Analysis;
Digital Medicine - Cytopathology and Anatomopathology.
IMAGE SERVICES
Clinoson – Ultrasound/Ultrasonography, Mammography, Magnetic Resonance Imaging;
